Table of Contents
- Introduction
- What is Nafithromycin?
- Scientific Innovation and Mechanism of Action
- Why India Needs Nafithromycin Now
- Antimicrobial Resistance: A Growing Crisis
- Clinical Trials and Efficacy Data
- Economic and Strategic Significance for India
- Government and Regulatory Support
- Role in Strengthening India’s Pharma R&D Ecosystem
- Global Demand and Export Potential
- Challenges in Implementation and Usage
- Expert Opinions and Endorsements
- The Road Ahead: Future Outlook
- Conclusion
1. Introduction
India is poised to make a significant breakthrough in its healthcare and pharmaceutical sectors with the imminent launch of Nafithromycin, a novel macrolide antibiotic. Developed entirely within the country, this indigenous drug addresses the rising threat of antimicrobial resistance (AMR), particularly in treating community-acquired bacterial pneumonia (CABP). As global health systems struggle with ineffective treatments due to resistant bacteria, Nafithromycin emerges as a timely and potent solution.
2. What is Nafithromycin?
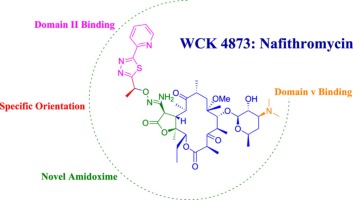
Nafithromycin is a next-generation macrolide antibiotic that promises rapid, effective, and short-course treatment against CABP and other respiratory tract infections. Engineered by the Indian pharmaceutical company Wockhardt, it features a three-day dosing regimen, enhancing patient compliance and therapeutic outcomes.
3. Scientific Innovation and Mechanism of Action
This antibiotic belongs to a new class of macrolides, possessing a structurally modified lactone ring. Nafithromycin exhibits improved binding to bacterial ribosomes, thereby effectively halting protein synthesis in resistant strains of Streptococcus pneumoniae and Haemophilus influenzae. The innovation lies in:
- Strong tissue penetration
- Extended half-life
- Reduced potential for cross-resistance
These properties make it ideal for outpatient settings and low-resource healthcare systems.
4. Why India Needs Nafithromycin Now
India records one of the highest burdens of respiratory infections and antibiotic resistance worldwide. The widespread misuse and overuse of antibiotics have rendered many traditional treatments ineffective. Nafithromycin offers a targeted and strategic intervention, aligning with the country’s broader public health objectives.
5. Antimicrobial Resistance: A Growing Crisis
According to WHO, AMR is expected to cause 10 million deaths annually by 2050 if no action is taken. In India, the problem is worsened by:
- Over-the-counter availability of antibiotics
- Incomplete treatment courses
- Lack of public awareness
The table below summarizes the top AMR threats in India:
| Pathogen | Associated Infections | Resistance Level | Alternative Treatments |
| Streptococcus pneumoniae | Pneumonia, Meningitis | High | Limited |
| Escherichia coli | Urinary Tract Infections | Moderate to High | Carbapenems, Colistin |
| Klebsiella pneumoniae | Bloodstream and lung infections | Very High | Carbapenems (limited success) |
| Mycobacterium tuberculosis | Tuberculosis | High (MDR/XDR) | Linezolid, Bedaquiline |
6. Clinical Trials and Efficacy Data
Nafithromycin has successfully completed Phase II and Phase III trials in India. Results have shown:
- 90% clinical cure rate in CABP
- Lower incidence of adverse effects
- No significant drug-drug interactions
Its pharmacokinetics allow once-daily dosing, a feature especially beneficial for adherence in rural and resource-limited areas.
7. Economic and Strategic Significance for India
The indigenous development of Nafithromycin reduces dependence on expensive imported medications. It also:
- Supports the ‘Make in India’ campaign
- Strengthens healthcare security
- Enhances global competitiveness in pharmaceutical innovation
In financial terms, India saves foreign exchange and creates employment in R&D and manufacturing.
8. Government and Regulatory Support
The Central Drugs Standard Control Organization (CDSCO) is in the final stages of approving Nafithromycin. Additionally:
- The National List of Essential Medicines (NLEM) may soon include it
- Customs duties on inputs have been waived to reduce costs
- The NPPA is expected to regulate its Maximum Retail Price (MRP) to ensure affordability
9. Role in Strengthening India’s Pharma R&D Ecosystem
Nafithromycin’s journey showcases India’s evolving pharmaceutical capabilities. The project benefitted from:
- Government-funded R&D incentives
- Academic partnerships
- A national action plan against AMR
This breakthrough marks a shift from generics manufacturing to original drug discovery in India.
10. Global Demand and Export Potential
Nafithromycin has significant export potential, especially to:
- Southeast Asia
- Sub-Saharan Africa
- Latin America
These regions share India’s burden of infectious diseases and antibiotic resistance. The drug’s short regimen and cost-effectiveness are particularly attractive to low- and middle-income countries (LMICs).
11. Challenges in Implementation and Usage
Despite its promise, several challenges remain:
- Risk of overprescription or misuse
- Need for robust antimicrobial stewardship programs
- Training healthcare providers on proper administration
- Resistance surveillance infrastructure
12. Expert Opinions and Endorsements
Medical professionals are optimistic. Dr. Randeep Guleria, former AIIMS Director, remarked:
“Nafithromycin is a timely innovation. Its short course and efficacy against resistant bacteria make it a vital tool in outpatient care.”
Dr. Priya Mehta, a microbiologist, added:
“We must couple this breakthrough with strong diagnostic support and public education to maximize its impact.”
13. The Road Ahead: Future Outlook
If approved and effectively deployed, Nafithromycin may pave the way for:
- Inclusion in global treatment guidelines
- Expansion of indication to skin, soft tissue, or ENT infections
- Licensing agreements with international agencies (e.g., WHO, GAVI)
- Development of combination therapies
14. Conclusion
Nafithromycin is not just a scientific breakthrough but a symbol of India’s rising leadership in healthcare innovation. By addressing a critical need with a domestically developed solution, India sets a precedent in fighting AMR and protecting public health.
With supportive policies, strategic implementation, and global collaboration, Nafithromycin could transform how the world approaches antibiotic resistance. Its success story can inspire more homegrown solutions for complex global health challenges.